Steps take the unbalanced equation and make a note of the elements present in each side of the equation. Then add coefficients to the atoms on each side of the equation to balance them with the same atoms on the other side.
To balance a chemical equation start by writing down the number of atoms in each element which is listed in the subscript next to each atom.

How to balance chemical equations easy steps.
How to balance chemical equations step 1.
Repeat for the other elements.
The net charge must be the same on each side of the.
How to balance chemical equationsexplanation and example 1.
2na cl 2 2nacl.
To do this keep in mind a subscript indicates the number of atoms.
Balancing chemical equations in five easy steps balancing chemical equations is a core skill in chemistry.
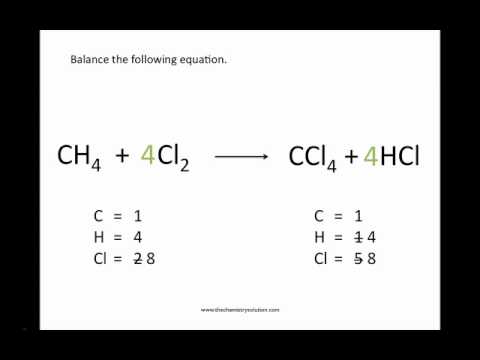
Now count the number of molecules of each element present on both sides of the equation.
In this video youll learn the basics for balancing equation with examples and.
Write the number of atoms.
Identify each element found in the equation.
In the above equation we have 2 sodium na and 2 chlorine cl on both lhs and rhs hence the equation is said to be balanced.
The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow.
For example o 2 has 2 atoms of oxygen.
Here comes the task of balancing the chemical equations.
Steps of balancing a chemical equation.
The number of atoms of each type of atom must be the same on each side of the equation once it has.
Identify the products and reactants.
Balance the first element.
What is the net charge on each side of the equation.
An equation is said to be balanced when there are equal number of molecules for each elements on both sides of the equations.
Fe o 2 fe 2o 3.
While balancing the equations you should only.

Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrzzthji892ajemhqr2ulplzemahgnufuymmpxw2k9rghekfyv Usqp Cau





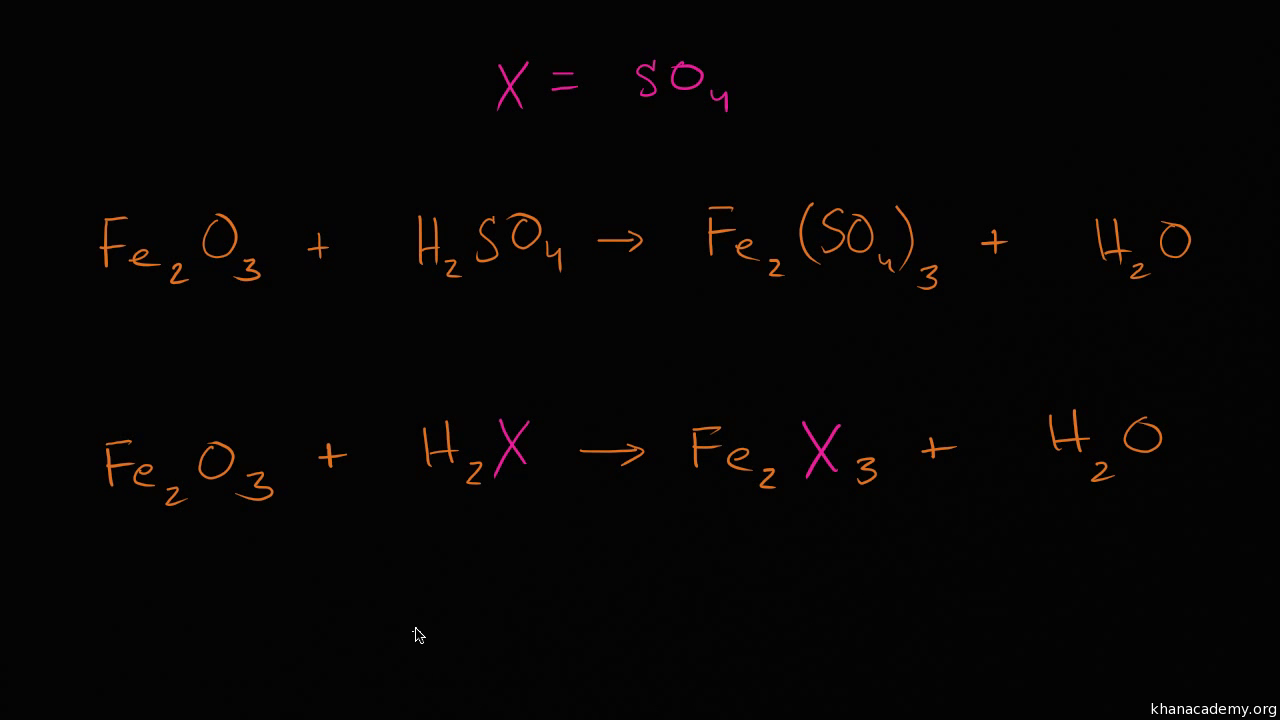

/ChemicalEquation-58dbe91d5f9b584683fa2f82.jpg)
No comments:
Post a Comment